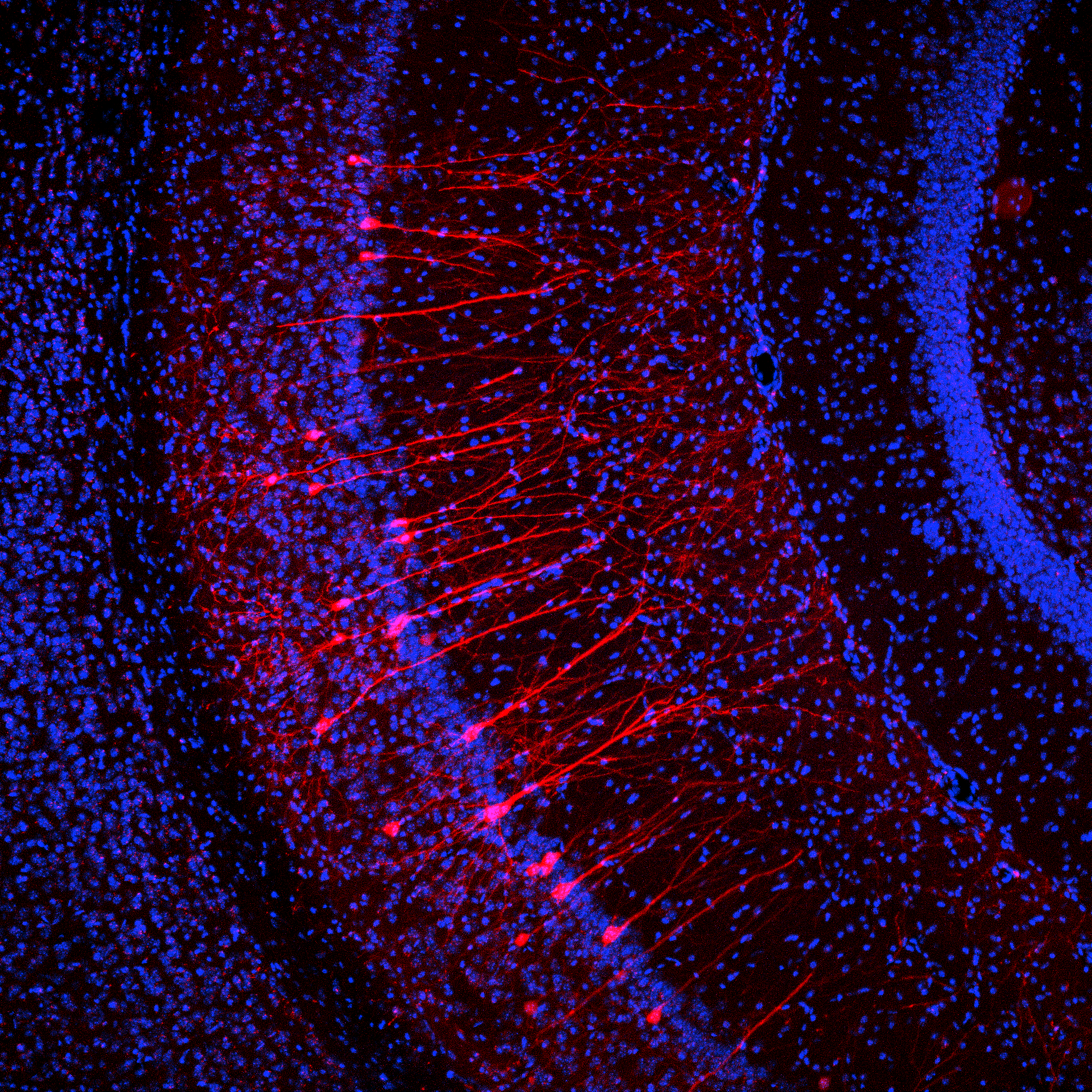
Publications

Dissociation of reinforcement and locomotor effects of cocaine in Pitx3 deficient mice
Beeler JA, Cao ZFH, Kheirbek MA, Zhuang X (2009)
Neuropsychopharmacology
Both the dorsal and ventral striatum have been demonstrated to have a critical role in reinforcement learning and addiction. Dissecting the specific function of these striatal compartments and their associated nigrostriatal and mesoaccumbens dopamine pathways, however, has proved difficult. Previous studies using lesions to isolate the contribution of nigrostriatal and mesoaccumbens dopamine in mediating the locomotor and reinforcing effects of psychostimulant drugs have yielded inconsistent and inconclusive results. Using a naturally occurring mutant mouse line, aphakia, that lacks a nigrostriatal dopamine pathway but retains an intact mesoaccumbens pathway, we show that the locomotor activating effects of cocaine, including locomotor sensitization, are dependent on an intact nigrostriatal dopamine projection. In contrast, cocaine reinforcement, as measured by conditioned place preference and cocaine sensitization of sucrose preference, is intact in these mice. In light of the well-established role of the nucleus accumbens in mediating the effects of psychostimulants, these data suggest that the nigrostriatal pathway can act as a critical effector mechanism for the nucleus accumbens highlighting the importance of intrastriatal connectivity and providing insight into the functional architecture of the striatum.
Adenylyl cyclase type V contributes to corticostriatal plasticity and striatum dependent motor learning
Kheirbek MA, Britt JP, Beeler JA, Ishikawa Y, McGehee DS, Zhuang X (2009)
Journal of Neuroscience
Dopamine (DA)-dependent corticostriatal plasticity isthoughtto underlie incremental procedural learning. A primary effector of striatal DA signaling is cAMP, yet its role in corticostriatal plasticity and striatum-dependent learning remains unclear. Here, we show that genetic deletion of a striatum-enriched isoform of adenylyl cyclase, AC5 knock-out (AC5KO), impairs two forms of striatum-dependent learning and corticostriatal synaptic plasticity. AC5KO mice were severely impaired in acquisition of a response strategy in the cross maze, a striatum-dependent task requiring a correct body turn to find a goal arm. In addition, AC5KO mice were impaired in acquisition of a motor skill, as assessed by the accelerated rotarod. Slice electrophysiology revealed a deficit in corticostriatal long-term depression (LTD) after high-frequency stimulation oftissuefrom AC5KOmice. LTD was rescued by activation of either presynaptic cannabinoidtype 1 (CB1 ) receptors or postsynaptic metabotropic glutamate receptors (mGluRs), suggesting a postsynaptic role of AC5– cAMP, upstream of endocannabinoid release. In striatopallidal-projecting medium spiny neurons, DA D2 receptors are negatively coupled to cAMP production, and activation of these receptors is required for endocannabinoid release and corticostriatal LTD. Recordings from striato pallidal neurons indicated that this is mediated by AC5, because coactivation of D2 and mGluRs could induce LTD in wild-type but not in AC5KO neurons. To further examine the role of cAMP in corticostriatal plasticity, we elevated cAMP in striatal neurons of wild-type mice via the recording electrode. Under these conditions, corticostriatal LTD was eliminated. Together, these data suggest an AC5– cAMP– endocannabinoid–CB1 signaling pathway in corticostriatal plasticity and striatum-dependent learning.
